
Neonatal sepsis is a widespread issue worldwide, and the current standard treatments are becoming less effective due to the combined challenge of high mortality rates and increasing antimicrobial resistance.
There is a need to conduct pragmatic trials that can provide valuable insights for managing neonatal infections in low and middle-income countries. Adaptive trials offer the most efficient design: these trials support adaptations and other necessary modifications to improve clinical practices and enhance the evidence base for neonatal infection management.
What is SNIP-AFRICA?
Severe Neonatal Infection Adaptive Platform trials in Africa
SNIP-AFRICA aims to target neonatal sepsis by establishing a clinical research network and architecture to implement adaptive platform trials in sub-Saharan Africa, responding to the urgent need for improved treatment of childhood infection in an era of increasing antimicrobial resistance.
SNIP-AFRICA will achieve its aim by bringing together partners from the global North and sub-Saharan Africa, including several partners with experience in designing and running adaptive platform trials and complex randomised controlled trials in sub-Saharan Africa. The project therefore builds on existing expertise and capacity to enable an innovative response to the major threat to child health of antimicrobial resistance through a new architecture and extended network.
Ultimately, SNIP-AFRICA aims to trigger a paradigm shift in interventional research in severe childhood infection and to equip a network of sub-Saharan African institutions and researchers to conduct innovative, efficient, targeted research in this area.
What are SNIP-AFRICA’s goals?
The project’s goals are:
- To create a sustainable platform, spanning the global North and sub-Saharan Africa, to conduct adaptive trials for treating severe childhood infections
- To implement an adaptive trial to determine the best antibiotic regimens for the treatment of infants with neonatal sepsis in sub-Saharan Africa within the ‘drug-regimen’ domain
- To conduct up to two pharmacokinetic studies within the ‘dose domain’ to support incorporation of antibiotics of interest at optimal neonatal doses into the platform
- To collect data on current clinical management, healthcare utilisation and bacterial epidemiology among hospitalised infants with neonatal sepsis
- To provide training opportunities on conducting adaptive platform trials to researchers and clinicians in sub-Saharan Africa
- To involve community representatives, clinicians, and regulators to ensure efficient and ethical implementation of adaptive trials through the platform.
What will SNIP-AFRICA do?
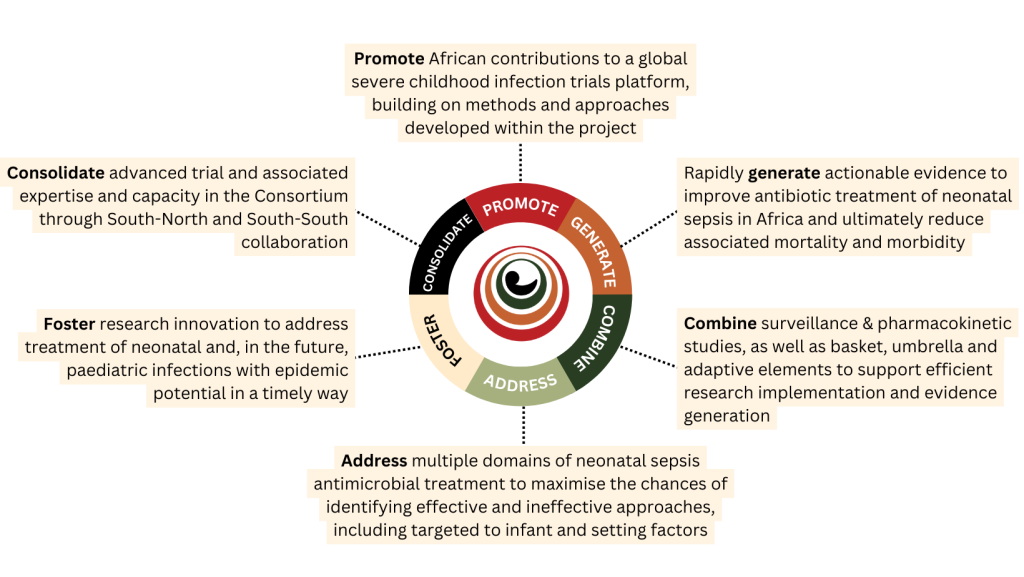

The SNIP-AFRICA project (101103201) is supported by the Global Health EDCTP3 and its members (the European Union and the EDCTP Association).