
Penta: new visual identity

c4c Consortium selects inaugural Research Portfolio
Immunoglobulin-like Domain of HsFcμR as a Capture Molecule for Detection of Crimean-Congo Hemorrhagic Fever Virus- and Zika Virus-Specific IgM Antibodies
Incidence of switching to second-line antiretroviral therapy and associated factors in children with HIV: an international cohort collaboration
Severe haematologic toxicity is rare in high risk HIV-exposed infants receiving combination neonatal prophylaxis.
Prevalence and clinical outcomes of poor immune response despite virologically suppressive antiretroviral therapy among children and adolescents with HIV in Europe and Thailand: cohort study
The use of polymyxins to treat carbapenem resistant infections in neonates and children
Effects of an antimicrobial stewardship intervention on perioperative antibiotic prophylaxis in pediatrics
Reactivity of routine HIV antibody tests in children with perinatally acquired HIV-1 in England: cross-sectional analysis
Accelerated aging in perinatally HIV-infected children: clinical manifestations and pathogenetic mechanisms

Global Health Festival, Padova, 5th-7th April
Adult dolutegravir 50mg film-coated tablets in children living with HIV weighing 20 to <25 kg

REACH: New website is online!

SMILE Clinical Trial: 100th Patient Enrolled
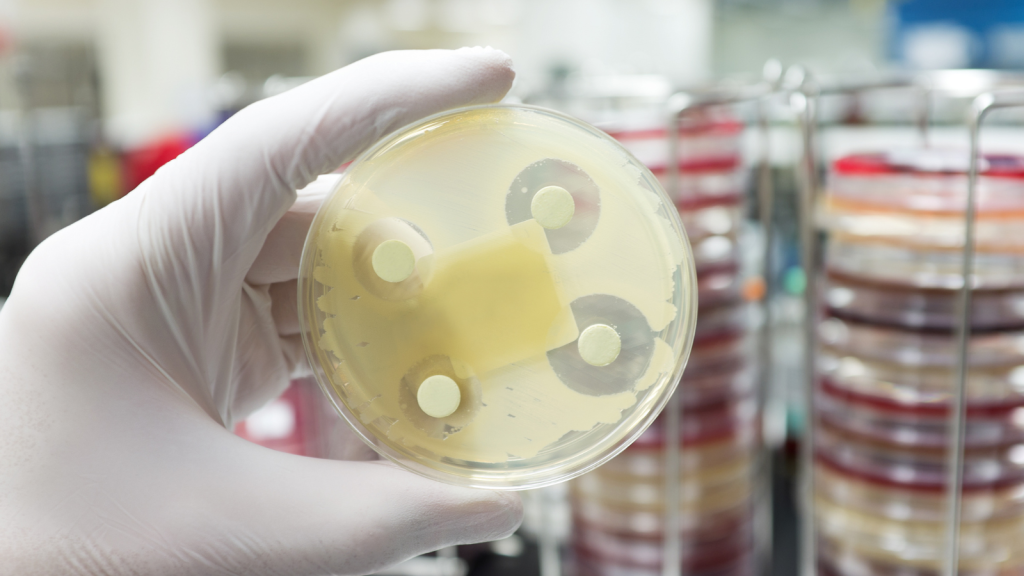
🇬🇧 GARDP and Penta partner to accelerate the development of children’s antibiotics to tackle AMR

Launch of VALUE-Dx in Madrid marked with press release
